 Valued at 9,400,000€, Bionure is overfunding on Capital Cell, raising more than 975,422€ from over 210 investors for 9.27% equity for its neuroprotector and myelin regenerator drug which treat Multiple Sclerosis.
Valued at 9,400,000€, Bionure is overfunding on Capital Cell, raising more than 975,422€ from over 210 investors for 9.27% equity for its neuroprotector and myelin regenerator drug which treat Multiple Sclerosis.
Bionure is a biotech-based company that is developing a new drug for the treatment of multiple sclerosis, a disease that affects about 2.5 million people worldwide with an annual cost of about $ 43,000 per patient and to date has no cure. In Spain alone, there are 47,000 people affected, and about 600,000 more in the rest of Europe. It is estimated that the market for a chronic regenerative drug such as BN201 would be about $ 1.2 billion. At present, there is no other drug that is both neuroprotective and regenerating, which makes the BN201 a potential absolute dominator of the market.

 The discovery and development of the BN201 is led by Dr. Pablo Villoslada and Dr. Albert G. Zamora who combine more than 40 years experience in research and innovation management.
The discovery and development of the BN201 is led by Dr. Pablo Villoslada and Dr. Albert G. Zamora who combine more than 40 years experience in research and innovation management.
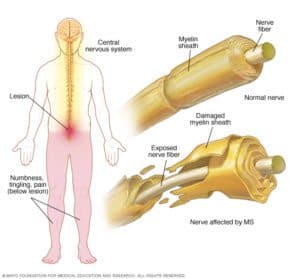
MS is a disease in which one’s immune system attacks the nervous system, degrading the layer of Myelin that covers the nerves and negating their proper functioning. These attacks occur over time, affecting different parts of the nervous system randomly (motor, sensory, linguistic, etc.), generating different symptoms in each patient. Despite not following an identical pattern in all patients, the degradation is irreversible in 100% of cases and most MS patients end up wheelchair -bound. Currently, after traffic accidents, MS is the second leading cause of disability in young adults.
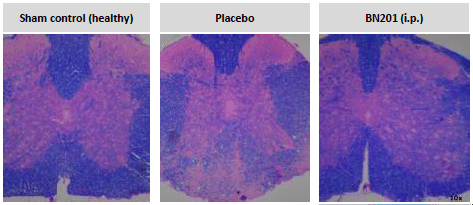
Bionure has identified and is developing BN201, a new drug that has the dual ability to protect neurons and regenerate damaged myelin, making it the first drug with the potential to completely halt the progression of multiple sclerosis. This drug uses a completely new mechanism of action (“first-in-class”) and after five years of research is already prepared to begin trials in humans, offering hope to MS patients, as well as other diseases neurodegenerative diseases.
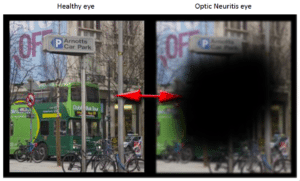 Initially BN201 will target patients with Acute Optic Neuritis (NOA), which is usually the first manifestation of Multiple Sclerosis. The main reason to start studies in this “subgroup” of MS is that Bionure has been designated as an orphan disease (an illness with an incidence of <5 / 10,000 inhabitants and without any treatment) and this makes legal procedures Reduce enormously and allow to extend the patent up to 5 additional years . From the strategic point of view, this substantially reduces the risk of the project, making it even more attractive. Patients with NOA suffer from pain in the eye and loss of vision, which may be partial (a black dot in the center of the field of view) or total. Once the BN201 has demonstrated its effectiveness for NOA in patients (completed phase II), its positioning for other variants of MS as well as other neurodegenerative diseases can be developed in a much simpler, faster and safer way.
Initially BN201 will target patients with Acute Optic Neuritis (NOA), which is usually the first manifestation of Multiple Sclerosis. The main reason to start studies in this “subgroup” of MS is that Bionure has been designated as an orphan disease (an illness with an incidence of <5 / 10,000 inhabitants and without any treatment) and this makes legal procedures Reduce enormously and allow to extend the patent up to 5 additional years . From the strategic point of view, this substantially reduces the risk of the project, making it even more attractive. Patients with NOA suffer from pain in the eye and loss of vision, which may be partial (a black dot in the center of the field of view) or total. Once the BN201 has demonstrated its effectiveness for NOA in patients (completed phase II), its positioning for other variants of MS as well as other neurodegenerative diseases can be developed in a much simpler, faster and safer way.
Before linking with Capital Cell, Bionure already received €7.5M of investment, both private and public. This round involves all current Bionure partners, including family offices and key figures in the Catalan industry: Reig Jofre (and its president Ignasi Biosca), the Monràs family (formerly Banc Sabadell), the Peris family (Salvat Laboratories ), Uriach family (Uriach Group), Prous family (Prous Institute for Biomedical Research), Pharmaphenix, Meeting Pharma, Galenicum, etc. These partners invest more than half of the round (approx €614K).
________________________________________________
______________________________________________-
And an update…. Barcelona-based Aromics, a privately-owned development stage pharmaceutical company founded in 2005, focuses on developing novel first-in-class therapies for the treatment of human diseases, particularly oncology and infectious diseases. The biotech firm’s campaign has raised over 281,910€ from 30 investors for 5.66% equity toward its 300K€ goal on Capital Cell.
Our goal is to cover first part of this financial requirements with the goal to complete preclinical regulatory package, foreseen for the second quarter of 2017,” according to Aromics. “Part of the 500,0000 euros required will be covered through the crowdfunding with CapitalCell (300.000 euros at a minimum ticket of 1.000 euros), and will be completed with the co-investment of the current shareholders and the support of R&D grants already achieved by the company for the project.”
Cofounded by CEO Dr. Carme Plasencia, CFO and Chairman of the Board Narcís Clavell and Immaculada Dalmau and now valued at 5M€, the company has already achieved several R&D awards from international, European, national and local public organizations, including the European Union, NCI, NIH, ICEX, CDTI, ENISA, ACC1Ó and MINECO.
To recap, Aromics’ current business model is to move validated inventive findings (protected under granted patents) up to early clinical stages (first-in-man efficacy Phase II). After completing clinical efficacy proof is foreseen, a licensing out, co-development agreement or direct product sale to pharmaceutical industry, according to the campaign. Revenues will come from dealmaking including upfront and milestone payments and royalty tiers. Usual multiply factor for similar investments are of x6.
The company is featuring NAX035, a first-in-class, selective and potent compound for the treatment of cancer. The novel drug is currently completing regulatory pre-clinics compelling all the data required by the European Medicine Agency, seeking the approval for conducting Phase IB/II (safety and efficacy) clinical testing in patients. Malignant mesothelioma has been selected as first indication for NAX035 to proceed into clinics due to the potency exhibited by the compound in this tumor type including both, pleural and peritoneal cancers. If effective, NAX035 will suppose a clear advance in the treatment of this rare cancer.
Why is this a business opportunity?
- Investing at this development stage represents an invest in a “new platform/strategy for the treatment of a refractory cancer”
- Mesothelioma incidence is increasing
- Current development will allow new treatment for malignant mesothelioma and other asbestos-related tumors ( lung, larynx and gastrointestinal among others). The investment will have a multiplier effect.
- Aromics has already succesfully developed with internal funds, a part of the preclinical development. The current drug supposes an advance up to clinics.
Details: Malignant mesothelioma is an aggressive tumor arising in the lining cells (mesothelium) of the pleural and peritoneal cavities, as well as in the pericardium and the tunica vaginalis. With a natural history of 7 to 9 months if untreated and less than five per cent 5-years survivors, is a bad prognosis tumor representing currently 0,3% of total cancer deaths. The World Health Organization (WHO) has recognized that all forms of mesothelioma are strongly associated with exposure to asbestos, a group of minerals presents in nature highly resistant to heat and corrossion. Due its physical properties, asbestos was widely used by the industry including: construction, minery, navy, textile, motor or plumbery among others. However, the use was limited some decades ago, being a material currently banned in fifty countries, as it is considered as a first level carcinogen.
Moreover, in those countries were has been already prohibited like EU, asbestos remains still a challenge as there are still many buildings and industrial elements containing asbestos that require removal and disposal. A high cost removal plan that also puts workers and the community at immense risk. WHO estimates that around 125 million people are currently professional and environmental exposed. From them 10% eventually develop mesothelioma.Currently, more than 100,000 people die due to asbestos. The burden of asbestos-related disease will keep rising over the next years and still represents a serious labour, public health and environmental problem. 29 days remain on Aromics campaign.
Have a crowdfunding offering you'd like to share? Submit an offering for consideration using our Submit a Tip form and we may share it on our site!

 Our goal is to cover first part of this financial requirements with the goal to complete preclinical regulatory package, foreseen for the second quarter of 2017,” according to Aromics. “Part of the 500,0000 euros required will be covered through the crowdfunding with CapitalCell (300.000 euros at a minimum ticket of 1.000 euros), and will be completed with the co-investment of the current shareholders and the support of R&D grants already achieved by the company for the project.”
Our goal is to cover first part of this financial requirements with the goal to complete preclinical regulatory package, foreseen for the second quarter of 2017,” according to Aromics. “Part of the 500,0000 euros required will be covered through the crowdfunding with CapitalCell (300.000 euros at a minimum ticket of 1.000 euros), and will be completed with the co-investment of the current shareholders and the support of R&D grants already achieved by the company for the project.”